New progress has been reported in the field of regulatory effect of ARC on the process of myocardial cell hypertrophy and myocardial apoptosis by Professor Pei-Feng Li and his Research Group of Cell Proliferation and Signal Transduction in IOZ. This work was recently published in three prestigious academic journals:Circulation Circulation.2008 Nov 25;118(22):2268-76.(IF=12.755),Molecular and Cellular Biology.2008;28:564-574.(IF=6.42), and Journal of Biological Chemistry.2008;283:5994-6004 (IF=5.581).
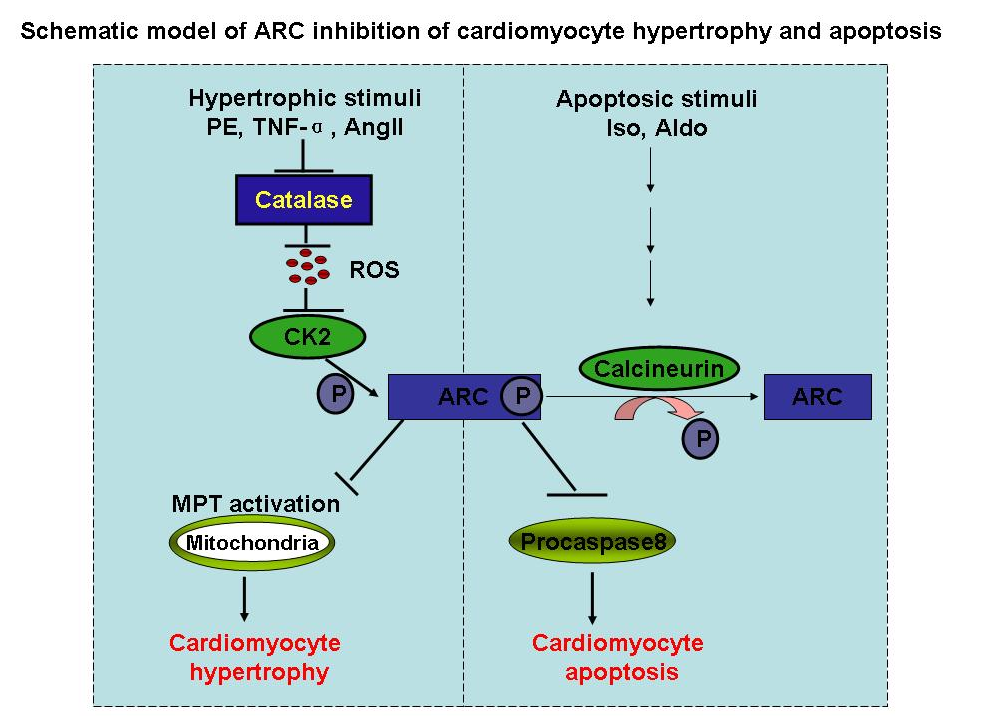
According to the introduction of researcher, apoptosis repressor with caspase recruitment domain (ARC) is abundantly expressed in the heart. It can directly bind to caspase-8 and inhibit caspase-8 activity. ARC inhibits caspase-8 activation by blocking the formation of death-inducing signaling complex. It binds to Fas and Fas-associated protein with death domain (FADD), thereby preventing death-inducing signaling complex formation. Further studies reveal that ARC also may elicit its function through other ways. It can interact with Bax, thereby inhibiting Bax activation and translocation to the mitochondria. It inhibits cytochrome c release and maintains mitochondrial membrane potential. In addition, ARC is regulated by protein kinase CK2 (CK2). CK2 can phosphorylate ARC at threonine-149 (T149). This phosphorylation enables ARC to translocate from the cytoplasm to the mitochondria where it directly binds to caspase-8 or caspase-2. ARC requires T149 phosphorylation to protect cells against oxidative stress?induced apoptosis. Strikingly, ARC phosphorylation by CK2 is constitutive, indicating the importance of phosphorylation for its function.
Despite the abundant expression of ARC, constitutive activation, and multiple ways to inhibit apoptosis, cardiomyocytes still undergo apoptosis under pathological conditions. Cardiomyocyte apoptosis can be triggered by a variety of stimuli such as oxidative stress, angiotensin II, and Fas ligand (FasL). Apoptosis is controlled by a complex interplay between proapoptotic and antiapoptotic factors. Under physiological conditions, this interplay remains in balance so that apoptosis is tightly controlled. However, the disruption of this balance under pathological conditions can result in apoptosis. Whether ARC can contribute to this imbalance when it loses its antiapoptotic function remains to be elucidated.
The β-adrenergic receptor agonist isoproterenol promotes cardiac remodeling, including apoptosis. Aldosterone can induce cardiomyocyte apoptosis both in vitro and in vivo. However, the molecular mechanisms by which isoproterenol and aldosterone induce cardiac apoptosis are not completely understood.
Calcineurin is a serine/threonine protein phosphatase. It is composed of a catalytic subunit (calcineurin A [CnA]) and a regulatory subunit (calcineurin B [CnB]). Calcineurin activation can be proapoptotic in cardiomyocytes. For example, coexpression of the constitutively active CnA together with CnB leads to apoptosis in cardiomyocytes. Isoproterenol induced cardiac apoptosis is related to calcineurin activation. Aldosterone-induced apoptosis also depends on the activation of calcineurin. The ARC phosphorylation site is a threonine residue. It is not yet clear whether calcineurin affects ARC in the apoptotic cascades.
The research work reveals that isoproterenol and aldosterone can induce ARC dephosphorylation through the activation of calcineurin. ARC dephosphorylation leads to its inability to inhibit caspase-8, thereby initiating apoptosis. Their data reveal a novel cardiac apoptotic pathway in which calcineurin dephosphorylates ARC. (By: Doctor Wei-Qi Tan)