The progressive functional decline in various organs with age contributes to a series of aging-related diseases, such as neurodegenerative diseases, cardiovascular diseases and diabetes. The diversity of cell types underlies the heterogeneity and complexity of tissues and organs, leading to distinct cellular and molecular features in different tissues and organs during aging. It is difficult to accurately reveal the cellular and molecular changes in different cell types during aging via traditional research techniques. In terms of aging interventions, caloric restriction (CR) has been shown to effectively delay aging in various species, but the exact mechanisms, especially the tissue- and cell type-specific molecular mechanisms, remain unclear.
Recently, scientists from the Institute of Zoology of the Chinese Academy of Sciences, Beijing Institute of Genomics of the Chinese Academy of Sciences, and Salk Institute for Biological Studies have worked jointly and established the first multi-tissue single-cell transcriptomic atlases in Rattus norvegicus undergoing aging and CR. Using the single-cell and single-nucleus transcriptome sequencing techniques, they drew a comprehensive roadmap across various tissues of rats undergoing aging and CR. They systematically evaluated the effects of aging and CR on different types of tissues and cells in the body at multiple dimensions, and revealed new molecular mechanisms of CR in delaying aging via the regulation of inflammatory responses in multiple organs. This study entitled "Caloric Restriction Reprograms the Single-Cell Transcriptional Landscape of Rattus Norvegicus Aging" is published online in Cell on February 27th, 2020.
The study shows that CR (a 70% calorie-restricted diet) since middle adulthood ameliorated the features of aging at the tissue, cellular and molecular levels and extended the lifespan of rats. The researchers drew the atlases for the transcriptional networks in different organs during aging and CR based on the transcriptome analyses of more than 200,000 single cells and nuclei from nine tissues in groups of young rats fed ad libitum, old rats fed ad libitum and old rats subjected to CR since middle adulthood. Based on these atlases, the researchers elucidated the effects of aging and CR on cell type composition, gene expression, core transcriptional regulatory networks and cell-cell communications in different tissues and organs of rats.
The researchers found that the aging-related changes in cell type composition occurred in all the tested tissues, more than half of which could be suppressed by CR. Notably, the numbers of immune cells (such as neutrophils and plasmocytes) were increased in most organs and tissues during aging, along with an increased ratio of pro-inflammatory macrophages to anti-inflammatory macrophages. These results suggest that increased inflammatory stress is a hallmark of aging across multiple tissues, and more importantly, that CR could reverse the abnormal changes in the ratio of immune cells. In terms of gene expression, the researchers detected aging-related changes in gene expression in different tissues and cell types. CR reversed the differential expression of more than a quarter of these aging-regulated genes, mainly those involved in inflammatory response and lipid metabolism. In particular, the expression of inflammatory factors such as S100a8 was upregulated in more than 40 cell types during aging and downregulated in more than 30 cell types by CR. Consistent with these findings, the high level of S100A8 in the serum of aged rats was decreased by CR, suggesting this protein as a potential novel biomarker of aging. Besides, the researchers identified that Ybx1, a regulator of mRNA transcription, splicing and translation, was downregulated during aging and upregulated by CR in more than 20 cell types. Using human and rat adipose-derived stem cells, the researchers further discovered that Ybx1 knockdown caused senescence phenotypes such as impaired cell proliferation. These results suggest that Ybx1 may function as a key molecular switch between the effects of aging and CR. In terms of intercellular communication, the researchers constructed a map of cell type-specific ligand-receptor pairs and found that the abnormally enhanced cell-cell communications during aging, especially those that trigger inflammatory responses, were mitigated by CR, which is consistent with the results obtained via the analyses of cell type composition and gene expression.
Taken together, Prof. Guang-Hui Liu and his colleagues successfully depict the cellular and molecular hallmarks of aging and CR rats at the multi-tissue level for the first time by combined application of a variety of technologies. This work systematically reveals that chronic inflammation is a common feature of aging across multiple organs and tissues in mammals, providing new cellular and molecular candidates as aging biomarkers. In addition, this study emphasizes the importance of the immune system in aging intervention by CR, laying a foundation for the development of therapeutic strategies against aging and aging-related diseases.
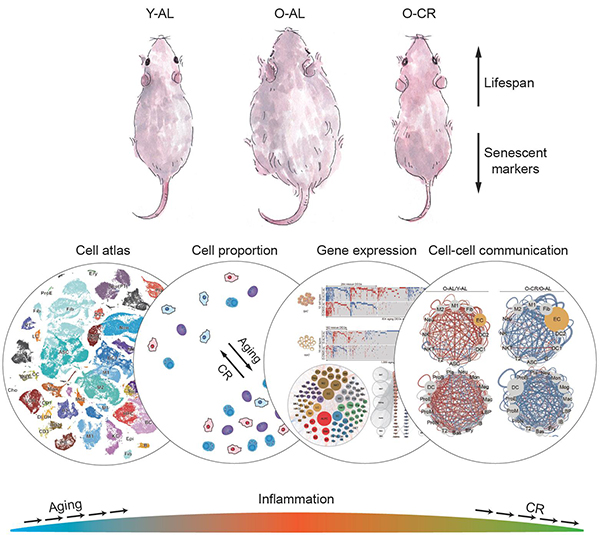
Figure 1. A Multi-Tissue Single-Cell Transcriptional Landscape of Rattus Norvegicus Undergoing Aging and Caloric Restriction.

