Zinc finger protein with KRAB and SCAN domains 3 (ZKSCAN3) is a member of the Kru?ppel-associated box domain zinc finger protein (KRAB-ZFP) family, with a SCAN domain in the N-terminus (SCAN-ZFPs). Emerging evidence has shown that ZKSCAN3 negatively regulates autophagosome formation by repressing the expression of autophagy-related genes. However, previous studies on ZKSCAN3 have been primarily based on tumor cell lines or animal models whereas the functions of ZKSCAN3 in normal human diploid cells was unclear.
The researchers from the Institute of Zoology of Chinese Academy of Sciences and Beijing Institute of Genomics of Chinese Academy of Sciences have worked jointly and published a study entitled “ZKSCAN3 counteracts cellular senescence by stabilizing heterochromatin” in Nucleic Acids Research on May 19th, 2020. The study for the first time reported that ZKSCAN3 functioned independent of its canonical function as a transcription repressor in the regulation of autophagy, but acted as a geroprotector to attenuate human stem cell senescence. Mechanistically, ZKSCAN3 maintained heterochromatin stability via the association with heterochromatin and lamina-associated proteins, thus repressing the aberrant activation of repetitive sequences.
The researchers first observed decreased ZKSCAN3 protein levels in replicatively, pathologically and physiologically aged human mesenchymal stem cells (hMSCs), suggestive of ZKSCAN3 as a potential regulator of hMSC aging. To further dissect the role of ZKSCAN3 in human stem cell aging, ZKSCAN3-knockout human embryonic stem cells (hESCs) and hMSCs were generated via CRISPR/Cas9-mediated gene editing and directed stem cell differentiation techniques. Loss of ZKSCAN3 exhibited no obvious effects on the self-renewal and differentiation abilities of hESCs, but accelerated hMSC senescence.
Conjoint analysis of Co-IP/MS, DamID-seq, ChIP-seq, ATAC-seq and RNA-seq revealed that ZKSCAN3 interplayed with nuclear lamina proteins (Lamin B1, LBR) and heterochromatin-associated proteins (KAP1, HP1a). ZKSCAN3 deficiency resulted in the detachment of genomic lamina-associated domains (LADs) from the nuclear lamina, loss of heterochromatin, more accessible chromatin status and aberrant transcription of repetitive sequences (LINE, SINE, Satellite family), thus leading to hMSC aging. Notably, overexpression of ZKSCAN3 or activation of endogenous ZKSCAN3 expression via CRISPRa system alleviated premature senescence phenotypes in aged hMSCs.
This study for the first time unveils that ZKSCAN3 functioned as a heterochromatin stabilizer, provides new insights into the functions of SCAN-ZFPs in epigenetic regulation, and points to ZKSCAN3 as a potential therapeutic target for delaying human stem cell aging and aging-related diseases in the future.
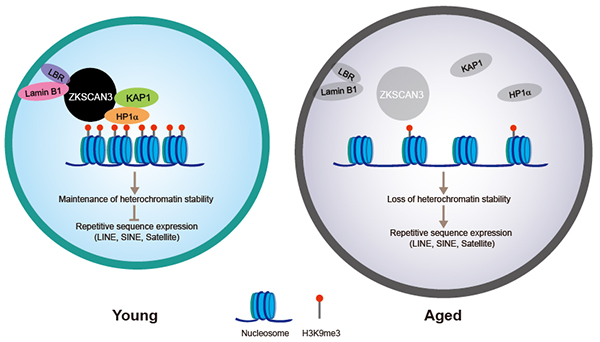
Figure. A model showing that ZKSCAN3 safeguards hMSCs from senescence by stabilizing heterochromatin.
link:https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkaa425/5840577

