The co-option between viruses and humans plays important roles during human evolution. Endogenous retroviruses (ERVs), belonging to long terminal repeat (LTR) retrotransposons, are a relic of ancient retroviral infection, fixed in the genome during evolution, comprising about 8% of the human genome. As a result of evolutionary pressure, most human ERVs (HERVs) accumulate mutations and deletions. Moreover, these enemies from ancient times are strictly repressed by host mechanisms such as epigenetic regulation. On the other hand, cellular senescence is an important hallmark of aging and aging-related diseases, during which, programmed epigenetic alterations play important roles. However, can endogenous retroviruses, or the enemies within, escape host surveillance during aging? And, if so, will they set the path to the grave of the cell or even the whole organism?
In this study entitled “Resurrection of endogenous retroviruses during aging reinforces senescence” in Cell published online on January 6, 2023, scientists from the Institute of Zoology and Beijing Institute of Genomics of the Chinese Academy of Sciences have collaborated to reveal that the youngest subfamily of ERV is awakened during aging, proposed a new theory of programed and contagious aging induced by resurrection of ERVs. Moreover, they found ways to control them: a multi-dimensional intervention strategy to block the reactivation and transmission of ERV to alleviate aging.
Using various aging models, including Hutchinson–Gilford progeria syndrome (HGPS) and Werner syndrome (WS), replicatively and physiologically senescent human mesenchymal progenitor cells (hMPCs) and human fibroblasts, as well as multiple organs from physiological and pathological aging models of mice, monkeys and humans, combined with multiple technologies, including whole genome profiling of RNA and DNA methylation, high resolution single molecule RNA/DNA-FISH, and highly sensitive digital PCR, researchers have found that epigenetic derepression (such as heterochromatin loss) leads to transcriptional activation of ERV, thereby increasing the translation of viral proteins, as well as the accumulation of viral-like particles (RVLPs) in senescent cells. On the one hand, the reverse transcripts of ERVs activate the cGAS-STING-mediated innate immune pathway, thereby eliciting inflammatory responses and accelerating cellular senescence. Moreover, RVLPs released by senescent cells can effectively transmit and amplify aging signals among organs, tissues and cells in a paracrine or humoral-mediated manner, thereby leading to the senescence of “infected” young cells. Furthermore, researchers have developed multiple effective intervention strategies to inhibit the reactivation of ERVs and eliminate viral particles, including CRISPR/dCas9 mediated gene editing systems that target the regulatory elements of ERVs, small molecule drugs that target reverse transcriptase, and neutralizing antibodies that target viral envelope proteins and other technologies. Each of these intervention strategies blocks a different step of the viral life cycle, such as ERV transcription, reverse transcription, and viral infection, to alleviate tissue and organismal aging.
This study provides evidence that aging-induced resurrection of endogenous retrovirus (AIR-ERV) is a hallmark and driving force of cellular, tissue and organismal aging. These findings provide fresh insights into aging mechanisms and lay the foundation of the theory of programmed, transmissible and intervenable aging. Moreover, this work opens avenues for establishing a scientific method for evaluating aging and developing clinical strategies to alleviate aging and aging-related diseases. Altogether, the resurrection of ERV may shed new light onto the "Pandora's box" during aging, which opens up a new scientific field, and paradoxically, brings new hope for preventing and treating aging-related diseases. In the future, more puzzles related to the activation of ERVs during aging need to be solved by continuous efforts of scientists via new techniques.
Link:https://doi.org/10.1016/j.cell.2022.12.017
Contact:
Guang-Hui Liu
Institute of Zoology Chinese Academy of Sciences
Tel: 86-64807852
E-mail: ghliu@ioz.ac.cn
Web: http://english.ioz.cas.cn/
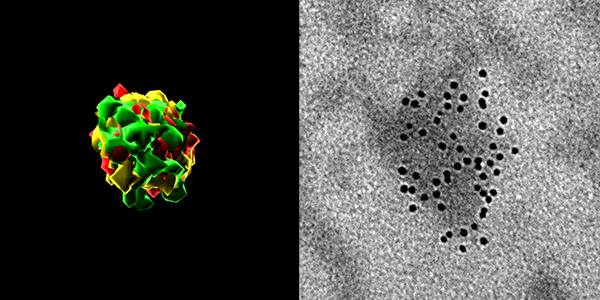
Figure 1. The accumulation of HERVK viral RNA (left) and viral-like particles (RVLP) in senescent human cells

