As the most prevalent chemical modification on eukaryotic mRNAs, N6-methyladenosine (m6A) is dynamically and reversibly regulated by corresponding methyltransferases (writers), binding proteins (readers) and demethylases (erasers). Why do we need such sophisticated regulation? Imagine the cells in our body as a bustling city, with m6A acting as a versatile traffic controller, guiding the flow of information — RNA, for its splicing, transport, stability, translation, etc. — in our cells. This cellular traffic controller is crucial for maintaining order in the city, affecting processes including embryonic development, tumorigenesis, and neurodegeneration. However, the potential regulatory role of m6A in the maintenance of tissue homeostasis during physiological aging has remained a mystery.
Recently, researchers have uncovered a new role for m6A in preserving the balance of our tissues as we age, through a collaborative study conducted by researchers from the Institute of Zoology and Beijing Institute of Genomics of the Chinese Academy of Sciences. This study entitled “m6A epitranscriptomic regulation of tissue homeostasis during primate aging” was published online in Nature Aging on April 6th, 2023. Using tissues from young and old cynomolgus monkeys as well as human stem cell derivatives, the researchers explored the dynamic landscapes of m6A across tissues during primate aging and revealed a novel role of the METTL3-m6A-NPNT axis in counteracting aging-associated skeletal muscle degeneration.
In this study, using physiologically aged nonhuman primates, the researchers illustrated m6A landscapes in three representative tissues susceptible to aging-related diseases, i.e., liver, skeletal muscle and heart via m6A sequencing. They revealed a positive correlation between m6A modification and gene expression homeostasis across tissues as well as tissue-specific m6A alterations during aging. Specifically, only aged skeletal muscles showed a reduction in global m6A abundance and METTL3 protein levels. Combined with further analysis in human stem cell-derived myotubes, researchers uncovered a protective role of METTL3 in counteracting skeletal muscle degeneration, pinpointed NPNT as a key downstream target of the METTL3-m6A axis in maintaining skeletal muscle homeostasis, and identified IGF2BP1 as a binding protein that stabilizes NPNT mRNA.
Now, picture METTL3 as the chief architect in charge of the cellular city's infrastructure. It works alongside NPNT to maintain the structural integrity of our muscles. When NPNT is reduced, our muscles weaken and deteriorate, much like a city's buildings crumbling over time. By boosting NPNT levels, however, the researchers were able to slow down muscle cell aging, highlighting the potential for new interventions to combat age-related disorders.
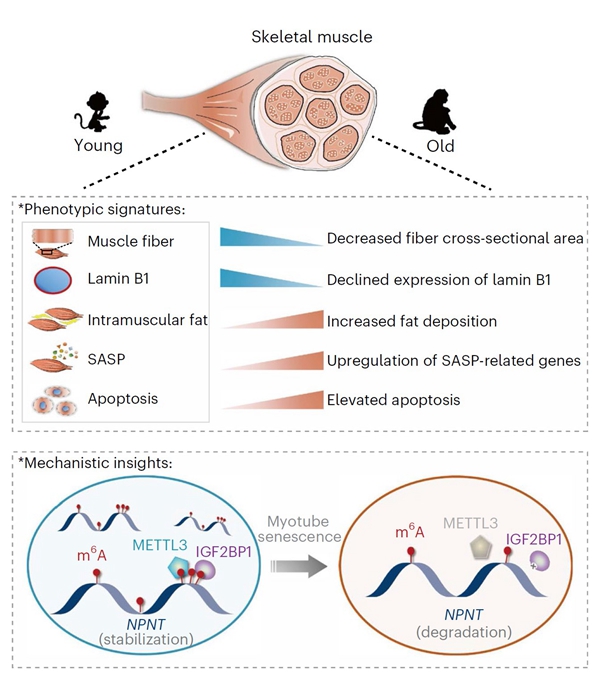
Figure1. A schematic diagram showing the phenotypic and epitranscriptomic signatures of primate skeletal muscle aging. ( Image by Zeming Wu)
Taken together, this study reveals dynamic m6A landscapes in maintaining homeostasis across tissues during primate aging and identifies a novel role of the METTL3-m6A-NPNT axis in counteracting skeletal muscle degeneration. These findings can be utilized to provide tissue-specific biomarkers for aging prediction and potential intervention targets to alleviate aging-associated disorders, such as sarcopenia, and ultimately helping us to maintain the vitality of our cellular cities as we grow older.
Figure 2. Image indicates that METTL3 promotes NPNT mRNA stability and counteracts skeletal muscle aging via m6A modification. (Image by Yizhu Wang)
Link:https://doi.org/10.1038/s43587-023-00393-2
Contact:
Guang-Hui Liu
Institute of Zoology Chinese Academy of Sciences
Tel: 86-64807852
E-mail: ghliu@ioz.ac.cn
Web: http://english.ioz.cas.cn/


