Spatial transcriptomics has emerged as a powerful tool for in situ analysis of gene expression within tissues. However, current technologies still face several challenges, including high costs, limited field of view, and low throughput, significantly hindering their application in large-scale tissue research and the analysis of complex biological processes.
Recently, Prof. ZHAO Fangqing's team from the Institute of Zoology, Chinese Academy of Sciences, developed a novel technology called MAGIC-seq. By integrating advanced grid-based microfluidic design, carbodiimide chemistry, and spatial combinatorial indexing, MAGIC-seq enhances detection throughput, reduces chip costs, and minimizes batch effects, while maintaining high resolution across large capture areas.
MAGIC-seq redefines the technological framework of spatial transcriptomics through its grid-based microfluidic chip design and optimized spatial encoding strategies. By forming "One combination, Multiple spots" through multiple-crossover reactions, MAGIC-seq significantly increases detection throughput and reduces chip fabrication costs, while maintaining high sensitivity. This design not only greatly enhances the economic feasibility of spatial transcriptomics research but also minimizes batch effects between different samples, which is particularly important when handling large numbers of samples.
Traditional microfluidics is limited in size and channel numbers. By introducing the concept of a "splicing chip," which adjusts grid spacing and uses multiple rounds of encoding to splice multiple capture grids together, they break through the channel limitations and achieves a balance between high resolution and a wide field of view. Researchers can customize the grid layout according to the shape and number of different samples, optimizing throughput or splicing chip shapes to better utilize spatial information.
The research team tested MAGIC-seq on various tissues, demonstrating superior detection sensitivity, sequencing efficiency, and data consistency compared to the most widely used 10x Visium platform and other methods. Using the splicing chip, they finely mapped mouse tissue slices across developmental stages, capturing the formation of organ structures and spatial gene expression changes, and identifying key genes influencing cerebellar development. Additionally, the team constructed a high-quality 3D spatial transcriptome map of the developing mouse brain, revealing cell and molecular distributions and uncovering dynamic tissue changes throughout development.
MAGIC-seq’s innovation and flexibility mark a significant advancement in spatial transcriptomics, positioning it as a vital tool for future research in this rapidly evolving field.
This study titled "Custom microfluidic chip design enables cost-effective three-dimensional spatiotemporal transcriptomics with a wide field of view" was published in Nature Genetics on September 10, 2024.
![]()
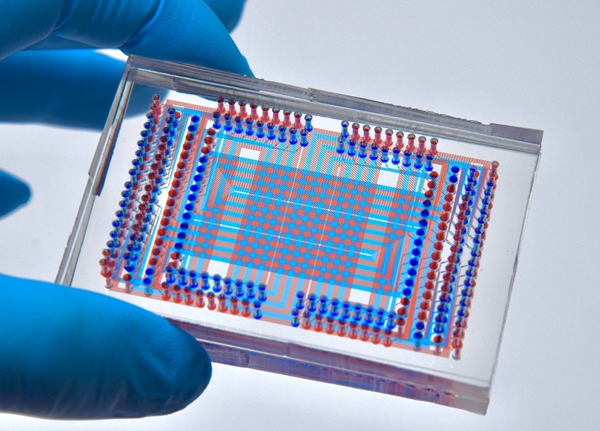
Fig. 1 Splicing grid chip for MAGIC-seq (Image by ZHAO Fangqing)
Article link: https://www.nature.com/articles/s41588-024-01906-4

